Temperature mapping study, qualification, validation and certification is very important for air cargo facilities, which handle pharmaceutical products. Temperature mapping study is an important requirement for any facility, which handles the storage and transportation of medicines, vaccines etc. This is to ensure that the facility is capable of maintaining the desired temperature of 2 to 8°C, 15 to 25°C etc. during storage and transportation.
Importance of temperature mapping study at Air cargo facilities
In the entire chain of Cold Chain Management of pharmaceutical products, airport handling is the weakest point where it is generally difficult to maintain the temperature. Within a span of a couple of hours, a medicine consignment has to undergo many loading and unloading sequences. This includes movements from the aircraft to the storage facilities at ambient temperature. Then it has to move to another aircraft or a reefer truck etc. Hence, the challenges are huge compared to other events in the entire chain of the cold chain.
 Challenges in Cold Chain at an Airport
Challenges in Cold Chain at an Airport
As an example, we will consider a consignment of medicines manufactured in Singapore and being transported to the USA through Dubai. The cargo has to be unloaded at Dubai and moved into a temporary storage facility within the airport. In summer months, the ambient temperature at Dubai is close to 50°C making it a huge challenge. As soon as we unload the consignment, we have to take proper measures to protect it from temperature. So, the ground staff has to immediately load them into temperature-controlled vehicles at the tarmac. This vehicle moves to the cold room somewhere at the airport. This cold room definitely is with a temperature suitable for these medicines.
-
Pharma Cold Room
The cold room for medicines is generally having a temperature between 2 to 8°C. The challenge with this cold room is the frequent door opening. It is easily possible to control door opening of a cold room at a pharma warehouse. However, at the airport, there are many cargo flights at short intervals. All of them might have a few consignments of pharma items. All of them come into the cold room and move out of the cold room frequently. This makes it impossible to have a controlled door opening sequence. This makes it difficult for stabilization of temperature inside the cold room or warehouse.
In order to improve this, we can provide various features including airlocks. Air insulation with two levels of airflow will improve performance. This is different from a normal pharma cold room, where only one level of air curtain is sufficient. The air insulation has to be automated so that as soon as the door opens, the air cushion starts working.
-
Pharma Warehouse

A pharma warehouse will have a temperature between 15 to 25°C. The challenges with the warehouse are similar to that of a cold room but less significant. The range of temperature is 10°C. Also, this temperature is much closer to the ambient than a walk-in cold room. Frequent door opening causes an inflow of temperature and humidity from the outside environment. In case you want to keep the humidity below 65%, you may consider installing dehumidifiers. (Normally pharmaceutical distributors maintain the humidity levels below 65%).
-
Pharma Cool cells
Individual Pharma cool cells with separate doors are comparatively at a lesser risk since the volume of a cool cell is only for storing one or two pallets at a time.

However, if a lot of cool cells without individual doors are arranged in a big cold room, the challenges are the same as that of a cold room. -
Transportation on Tarmac / Ramp
This is the biggest challenge of all the activities especially in Middle East countries where the ambient temperature goes up to 50°C. Different airports use a different type of temperature controlled vehicles for such purpose. The consignment may be inside a ULD (Unit Load Device) which has to directly go into the vehicle. One such specialized vehicle is Cool Dolly being used by different airports.
-
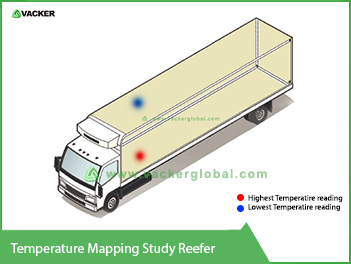
 Reefer trucks for transportation between local airports
Reefer trucks for transportation between local airportsIn many cases, it is possible that there will be a couple of local airports. In such a case, the cargo handling company moves the pharma goods at one airport and then transport to another local airport for loading into another aircraft. In such cases, they use temperature-controlled reefer trucks. Hence it is essential to carry out temperature mapping study on such trucks. These reefer trucks are different from normal reefer trucks. They are more suitable for smooth handling ULDs. Normal reefer trucks are suitable for loading and unloading using forklifts. These trucks will have rollers on the floors for easy movement of ULDs or pallets.
 We are proud to be part of different airlines for GDP certification process of Airlines
We are proud to be part of different airlines for GDP certification process of Airlines
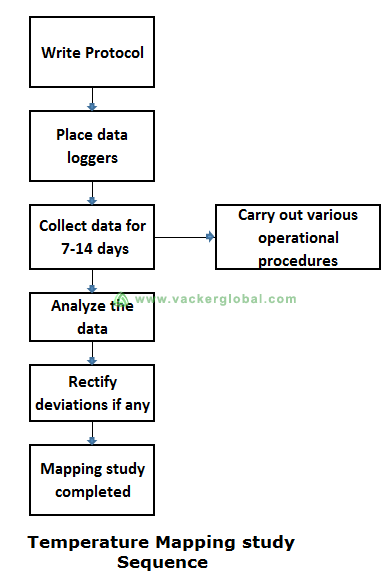
We have carried out the temperature mapping study and certification for different airport facilities. The entire process covers temperature mapping study in two seasons ie. Summer and winter. Winter mapping is less strenuous compared to summer mapping. In rare cases, customers require a study in the spring season too.
For all critical facilities, we carried out an initial trial test to save time and efforts. This helped the client to take corrective actions on time. If we go ahead with actual tests and find out the excursions, it might become close to the end of the season. This will make it difficult for clients. Hence, for temperature mapping study of such facilities, we always recommend a trial test to find out major anomalies if any. Sometimes, a slight change in settings of a thermostat was sufficient to correct major escalations.
 Procedure for temperature mapping study for airlines
Procedure for temperature mapping study for airlines
The test procedures are in line with the following international GDP specifications.
- Spec no. 1079 – The regulation of United States Pharmacopeial Convention for Good Storage and Distribution Practices for Drug Products.
- Spec no. 1118 USP – This is for Monitoring Devices for Time, Temperature, and Humidity.
- Spec no. 961, 2011 of WHO for Good Storage and Distribution Practices for Pharmaceutical Products
The specifications are general in nature for GDP and not specifically for airport facilities. Compared to normal pharma distribution warehouses, there are some operational differences at an airport. The main difference is that the storage time is lesser compared to normal warehouses or cold rooms. Secondly, the door opening is much more frequent. The test procedure and protocol have to consider these aspects.
For ambient pharma warehouses (15 to 25°C), the test duration is normally 7-10 days, with a maximum of 24 days. A pharmaceutical distributor stores the goods for weeks or months. However, this is not the case for air cargo facilities. At an air cargo facility, the goods are generally stored only for a couple of hours or maximum of a couple of days. We have to incorporate such practical considerations in the protocol for the temperature mapping study and qualification.
Temperature monitoring system for Air cargo Pharma facilities
As part of GDP processes, it is essential to record the temperature of these facilities. This requires a continuous monitoring system. As part of our services, we provide a complete solution for temperature mapping study and monitoring systems.
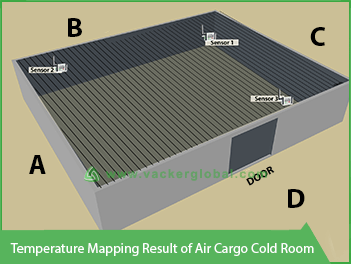
After carrying out the temperature mapping study, we provide the entire report. The report will show the hot and cold points inside the area and the recommended sensor locations. Depending upon the area of the facility, we will suggest the quantity of the sensors. You may note the following criteria while selecting a suitable temperature recording system.
- It may be better to select wireless (Radiofrequency, Zigbee or WiFI) devices. This is for the easier relocation of the sensors and will avoid wiring across the area.
- Based on your requirement you may select phone call, SMS or Email alerts.
- It should be possible to generate Mean Kinetic Temperature (MKT) value for any selected duration. Please note that you should be able to select a specific duration and calculate MKT value. This is different from the normal scenario in a warehouse of a distributor, where MKT calculation on a weekly or monthly basis is sufficient. In the case of storage at air cargo, there will be many consignments in the same room at the same time. As an example, we can consider that one ULD comes at 10.00 am and leaves at 6.00 pm on the same day. The consignor or consignee of this particular goods may ask the MKT value only for this particular duration.
- It should be ideally in compliance with 21 CFR Part 11.
- You may want to check the monthly charges. In order to evaluate different suppliers, you may check the total expenditure for a five-year period.
 Is humidity important for Air cargo Cold room and warehouse?
Is humidity important for Air cargo Cold room and warehouse?
Apart from temperature mapping study and temperature monitoring systems, we also provide complete dehumidification systems. Hence, the discussion always comes up whether humidity is important in an air cargo facility. As an airport handles products from numerous pharma manufacturers, a clear-cut answer is difficult. Each pharma manufacturer has its own specified requirement regarding the required humidity levels for storage of their products.
In view of the frequent door openings, generally, it is difficult to maintain humidity strictly under controlled conditions. In pharma warehouses of distributors, we have installed dehumidification systems to maintain humidity below 65% and even below 45%. However, maintaining 45% at a cargo facility will be a huge challenge. Otherwise, this will require dehumidifiers of huge processing capacity. Generally, the storage time at an airport is very short compared to a pharma distributor. Hence the humidity is generally not a major concern. Based on this you can check with your medicine manufacturer regarding the required humidity levels.
Cold chain validation and certification for airline cargo operators
Any airports handling cold chain goods such as medicines, vaccines, food etc. will have related assets. These assets include warehouses, cold rooms, cool cells, pallet handlers, trucks, vans, cool dollies, cool boxes etc. Depending on the goods being handled generally, there are three different temperature ranges viz. 15 to 25 ℃, 2 to 8 ℃ and below -18 ℃. The purpose of all these assets is to store the goods at the recommended temperature from the moment of unloading from an aircraft to loading.
The loading can be for local road transportation or to a different aircraft. From the moment of unloading to the next loading, each pallet may go through multiple transportation and storage assets. In order to ensure that all these assets are capable of maintaining the desired temperature, it is essential that all these assets are properly tested for performance.
Cold Chain certification of an airport cargo facility includes proper testing and validation of all such assets and their handling procedures. The assets are tested for their performance and validated for a certain period. The airport will have a standard operating protocol (SOP) which should be in line with the best GDP (Good Distribution Practices). An SOP is prepared based on the guidelines as well as the tested results of the assets. As an example, if you have a cool box on wheels which maintains the temperature for 60 minutes without power, it is essential to write an SOP reflecting the same.
Our services on Pharma certification of Aircargo facilities as per GDP
- Service Type: Temperature Mapping Study, qualification & Validation for pharma transportation through air cargo.
- Areas Served: The United States of America, United Kingdom, Canada, Australia, Newzealand, Europe, Germany, France, The United Arab Emirates, Saudi Arabia (Riyadh, Jeddah, Dammam, Jubail), Qatar, Oman, Bahrain, Kuwait, Kenya, Tanzania, Ethiopia, Rwanda, Uganda, Egypt, Jordan, Lebanon, India, Singapore, Pakistan.
- Service Offer: Temperature Mapping Study & Validation, Humidity Control Systems, Temperature Monitoring & alert Systems for Warehouse/Cold Stores/Vans/Boxes, Economic & Energy Saving Walk-In Cold Stores.
- Provider Name: VackerGlobal, 92438, Deira, Dubai, United Arab Emirates. Tel: +97142661144
- Service Description:
- Temperature mapping study, validation and certification of warehouses at Airports for storage of Food & Pharma.
- Temperature mapping study, validation and certification of cold rooms and walk-in freezers at Airports for storage of Food & Pharma.
- Temperature mapping study, validation and certification of medical refrigerators for Aircargo facilities.
- Temperature mapping study, validation and certification of thermally insulated passive boxes & active boxes for shipment of medicines through aircraft.
- Temperature mapping study, validation and certification of reefer trucks & refrigerated vans for transportation of food & Pharma goods at airports.
- Temperature mapping study, validation and certification of Thermally insulated pallet covers for covering pallets of food and pharma items for air shipment.
- Temperature mapping study, validation and certification of ULD (Unit Load Device) for shipment of food and medicines through the air.
Vacker Global is operating in the USA, Europe, MENA region, United Arab Emirates, India etc.